Our Solutions
Gas Denitration Technologies
Gas Denitration technologies, also known as DeNOx (nitrogen oxide removal) technology, are employed to reduce the emission of nitrogen oxides (NOx) from various combustion processes, particularly in power plants and industrial facilities. NOx compounds contribute to air pollution and are precursors to smog and acid rain. Here are two important denitration technologies:
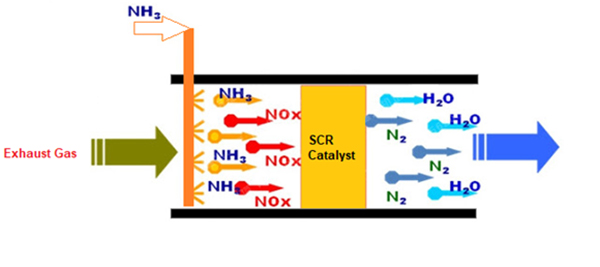
Selective Catalytic Reduction (SCR)
Selective catalytic reduction (SCR) denitration technology, is a process that converts nitrogen oxides (NOx) into nitrogen (N2) and water (H2O) with the help of a catalyst. This is achieved by adding a reductant, such as anhydrous ammonia (NH3), aqueous ammonia (NH4OH), or a urea (CO(NH2)2) solution, to a stream of flue or exhaust gas and reacting it onto a catalyst. The reaction produces nitrogen (N2) and carbon dioxide (CO2) in the case of urea use.
SCR technology is widely used in various industries, such as coal-fired boilers, incinerators, furnaces, and kilns plants. It is a mature and reliable technology, with a high efficiency that can reduce NOx by 70-95%.
The reactions involved in SCR denitration technology:
NO + NH3 → N2 + H2O
NO + NH4+ → N2 + 2H2O
NO + 1/2(NH2)2CO → 1/2(N2) + CO2 + 2H2O
Selective Non-Catalytic Reduction (SNCR)
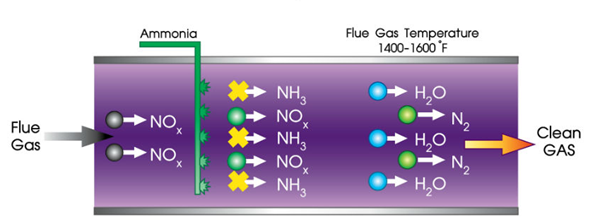
Selective Non-Catalytic Reduction (SNCR) is another denitration technology that reduces nitrogen oxide (NOx) emissions in the flue or exhaust gases. It is a chemical reaction process that uses ammonia or urea as a reducing agent to break down NOx into nitrogen (N2) and water (H2O) without the aid of a catalyst.
SNCR technology injects ammonia or urea solution into a high-temperature flue gas zone, where the reducing agent reacts with NOx to form N2 and H2O. This process is optimized by controlling the temperature and amount of reducing agent added to the flue gas. SNCR is typically used in boilers, furnaces, and other high-temperature combustion systems.
The general reaction for SNCR is the reduction of NOx with ammonia:
2NO + 2NH3 + O2 → 2N2 + 3H2O
Alternatively, a modified SNCR process called “Advanced SNCR” (ASNCR) can also occur, which involves the reduction of NOx with urea:
2NO + CO(NH2)2 + O2 → 2N2 + 2CO2 + 2H2O
In both reactions, the ammonia or urea is added to the flue gas at a temperature range of 800°C to 1100°C. The ammonia or urea reacts with the NOx in the flue gas, forming nitrogen, water vapour, and carbon dioxide. The reaction is highly exothermic, meaning it releases heat, and the high temperatures involved ensure that the reaction occurs quickly and efficiently.
Comparison between Selective Catalytic Reduction (SCR) and Selective Non-Catalytic Reduction (SNCR) Denitration Technologies
Catalyst Requirement
SCR: Requires a catalyst (commonly made of vanadium, titanium, or zeolites) to facilitate the reaction between ammonia or urea and nitrogen oxides.
SNCR: Does not require a catalyst; the reduction reaction occurs directly in the combustion chamber.
Temperature Range
SCR: Operates at lower temperatures (typically between 300°C to 400°C or 572°F to 752°F), making it effective in a broad range of combustion processes.
SNCR: Operates at higher temperatures (above 1,400°C or 2,552°F), suitable for applications where high temperatures are present.
NOx Removal Efficiency
SCR: Achieves high NOx removal efficiency, often exceeding 90%, making it suitable for facilities with stringent emission control requirements.
SNCR: Provides moderate to high NOx removal efficiency (typically between 30% to 70%), depending on application conditions.
Reaction Mechanism
SCR: Involves a chemical reaction facilitated by a catalyst, leading to the formation of nitrogen and water from nitrogen oxides and reducing agents.
SNCR: Involves a direct reaction between injected ammonia or urea and nitrogen oxides in the combustion chamber.
Suitability for High-Temperature Applications
SCR: Effective at lower temperatures, making it suitable for a wide range of combustion processes, including those with moderate temperatures.
SNCR: Specifically designed for high-temperature applications, making it suitable for processes where temperatures are elevated.
Rapid Reaction Time
SCR: Provides a relatively rapid reaction time due to the use of a catalyst.
SNCR: Also offers rapid reaction times, making it suitable for applications where immediate NOx reduction is required.
Cost Considerations
SCR: Typically involves higher capital and operational costs due to the use of a catalyst and associated system components.
SNCR: Generally considered more cost-effective due to the absence of a catalyst, making it attractive for certain applications.
Flexibility and Versatility
SCR: Suitable for large power plants and industrial facilities with a range of combustion processes, providing scalability and efficiency.
SNCR: Versatile and applicable to various combustion processes, especially where SCR may be challenging to implement.
Maintenance and Catalyst Life
SCR: Requires periodic maintenance of the catalyst but generally has a longer catalyst life compared to some other processes.
SNCR: Simpler in design and may have lower maintenance requirements, but the lack of a catalyst means it may require more frequent attention.
Selective Reduction
SCR: Selectively reduces nitrogen oxides without affecting other components of the flue gas.
SNCR: Also provides selective reduction, contributing to better environmental performance.
In summary, the choice between SCR and SNCR denitration technologies depends on factors such as temperature conditions, NOx removal efficiency requirements, cost considerations, and the specific characteristics of the combustion process in question. Each technology has its advantages and is selected based on the unique needs of the facility or industry.
