Scrubbers are critical devices used in various industries to remove pollutants from exhaust gases before they are released into the atmosphere. They employ various physical and chemical reaction to achieve this. Here, we delve into the chemical reaction mechanisms of wet and dry scrubbers, focusing on how they target and neutralize specific pollutants.
Wet Scrubbers
Wet scrubbers are highly effective devices used to remove pollutants from industrial exhaust streams by utilizing liquid solutions to capture and neutralize harmful gases and particulates. These systems are versatile and can handle a wide range of contaminants, including acidic gases, volatile organic compounds (VOCs), and particulate matter. The chemical reactions in wet scrubbers are detailed below:
1- Sulfur Dioxide (SO₂) Removal
Sulfur dioxide is a common pollutant generated by the combustion of fossil fuels. In wet scrubbers, SO2 is removed through its absorption in water followed by neutralization with alkaline agents.
Absorption and Hydrolysis:
When sulfur dioxide is absorbed into the water, it forms sulfurous acid (H2SO3):
SO2(g)+H2O(l)→H2SO3(aq)
Neutralization with Alkaline Solutions:
The sulfurous acid formed is then neutralized by an alkaline substance such as calcium hydroxide (lime) or sodium hydroxide (caustic soda):
- Using Calcium Hydroxide:
H2SO3(aq)+Ca(OH)2(aq)→CaSO3(s)+2H2O(l)
Further oxidation can convert calcium sulfite (CaSO3) to calcium sulfate (CaSO4):
CaSO3(s)+1/2O2(g)→CaSO4(s)
- Using Sodium Hydroxide:
H2SO3(aq)+2NaOH(aq)→Na2SO3(aq)+2H2O(l)
Na2SO3(aq)+1/2O2(g)→Na2SO4(aq)
2- Hydrochloric Acid (HCl) Removal:
Hydrochloric acid gas is another pollutant commonly found in industrial emissions. Wet scrubbers efficiently remove HCl by dissolving it in water and neutralizing it with alkaline solutions.
Absorption and Ionization:
Hydrochloric acid gas dissolves in water and ionizes:
HCl(g)+H2O(l)→H3O+(aq)+Cl−(aq)
Neutralization:
The hydronium ions (H₃O⁺) are neutralized by an alkaline agent, such as calcium hydroxide or sodium hydroxide:
- Using Calcium Hydroxide:
H3O+(aq)+Cl−(aq)+Ca(OH)2(aq)→CaCl2(aq)+2H2O(l)
- Using Sodium Hydroxide:
H3O+(aq)+Cl−(aq)+NaOH(aq)→NaCl(aq)+2H2O(l)
3- Removal of Other Acidic Gases
Wet scrubbers are also effective in removing other acidic gases, such as hydrogen fluoride (HF) and nitrogen oxides (NOx).
Hydrogen Fluoride (HF) Removal:
Hydrogen fluoride gas is absorbed and neutralized similarly to HCl:
HF(g)+H2O(l)→H3O+(aq)+F−(aq)
H3O+(aq)+F−(aq)+Ca(OH)2(aq)→CaF2(s)+2H2O(l)
Nitrogen Oxides (NOx) Removal:
Nitrogen oxides can be removed using a combination of water absorption and chemical reduction:
2NO2(g)+H2O(l)→HNO3(aq)+HNO2(aq)
The nitric acid (HNO₃) and nitrous acid (HNO₂) can then be neutralized by alkaline substances:
HNO3(aq)+Ca(OH)2(aq)→Ca(NO3)2(aq)+2H2O(l)
HNO2(aq)+Ca(OH)2(aq)→Ca(NO2)2(aq)+2H2O(l)
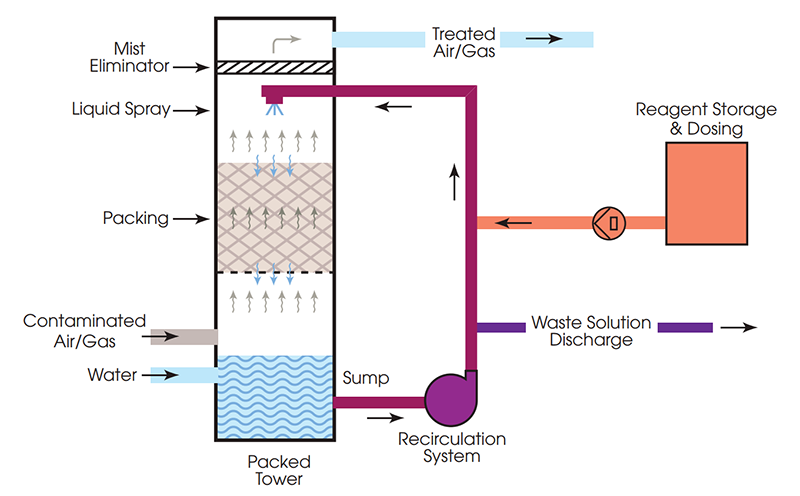
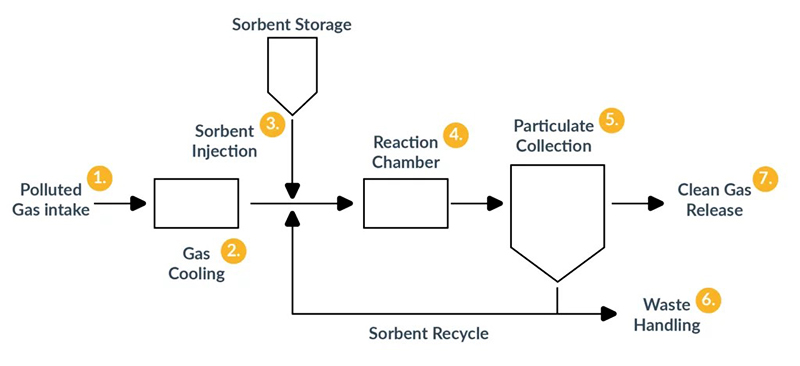
Dry Scrubbers
Dry scrubbers are an essential technology in controlling air pollution, particularly in industrial settings where liquid-based scrubbing systems may not be feasible. Unlike wet scrubbers, dry scrubbers use dry reagents or sorbents to capture and neutralize pollutants from exhaust gases. The chemical reactions in dry scrubbers are detailed below:
1- Sulfur Dioxide (SO₂) Removal
Sulfur dioxide is a common pollutant produced from the burning of fossil fuels. Dry scrubbers remove SO₂ through the reaction with alkaline sorbents such as calcium hydroxide (Ca(OH)₂) or sodium bicarbonate (NaHCO₃).
Reaction with Calcium Hydroxide:
SO2(g)+Ca(OH)2(s)→CaSO3(s)+H2O(l)
Further oxidation of calcium sulfite (CaSO₃) to calcium sulfate (CaSO₄) can occur:
CaSO3(s)+1/2O2(g)→CaSO4(s)
Reaction with Sodium Bicarbonate:
Sodium bicarbonate decomposes upon heating to form sodium carbonate (Na₂CO₃), which then reacts with SO₂:
2NaHCO3(s)→Na2CO3(s)+CO2(g)+H2O(g)
Na2CO3(s)+SO2(g)→Na2SO3(s)+CO2(g)
Further oxidation of sodium sulfite to sodium sulfate:
Na2SO3(s)+1/2O2(g)→Na2SO4(s)
2- Hydrochloric Acid (HCl) Removal
Hydrochloric acid gas can be effectively removed using dry scrubbing techniques involving alkaline sorbents.
Reaction with Calcium Hydroxide:
2HCl(g)+Ca(OH)2(s)→CaCl2(s)+2H2O(l)
Reaction with Sodium Bicarbonate:
HCl(g)+NaHCO3(s)→NaCl(s)+CO2(g)+H2O(g)
3- Removal of Other Acidic Gases
Dry scrubbers are also used to remove other acidic gases, such as hydrogen fluoride (HF) and nitrogen oxides (NOx).
Hydrogen Fluoride (HF) Removal:
- Reaction with Calcium Hydroxide:
2HF(g)+Ca(OH)2(s)→CaF2(s)+2H2O(l)
Nitrogen Oxides (NOx) Removal:
Nitrogen oxides can be reduced through a series of reactions, often involving ammonia (NH3) or urea as a reductant in selective non-catalytic reduction (SNCR) systems.
- Selective Non-Catalytic Reduction (SNCR) Reactions:
4NH3(g)+4NO(g)+O2(g)→4N2(g)+6H2O(g)
4NH3(g)+6NO2(g)→7N2(g)+12H2O(g)
By understanding the detailed chemical reactions in both dry and wet scrubbers, industries can optimize these systems for maximum efficiency and compliance with environmental regulations. The choice between dry and wet scrubbing systems depends on factors such as the type of pollutants, operational costs, and specific industrial requirements. Both technologies play a vital role in reducing harmful emissions and protecting air quality, contributing to a cleaner and healthier environment.
If you have any questions about our scrubbing technologies or need assistance in selecting the right system for your needs, please don’t hesitate to contact us. Our team of experts is here to provide you with tailored solutions and support for your pollution control requirements.

I just like the valuable information you provide for your articles. I’ll bookmark your weblog and check again right here frequently. I am reasonably certain I will learn plenty of new stuff right here! Best of luck for the following!