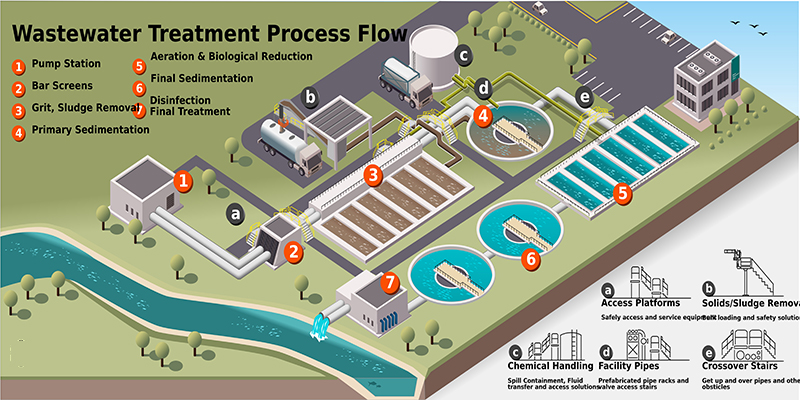
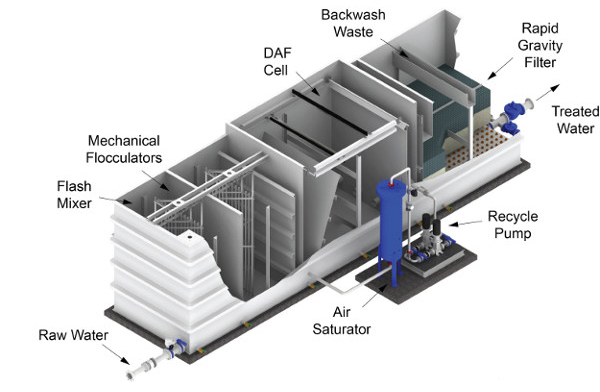
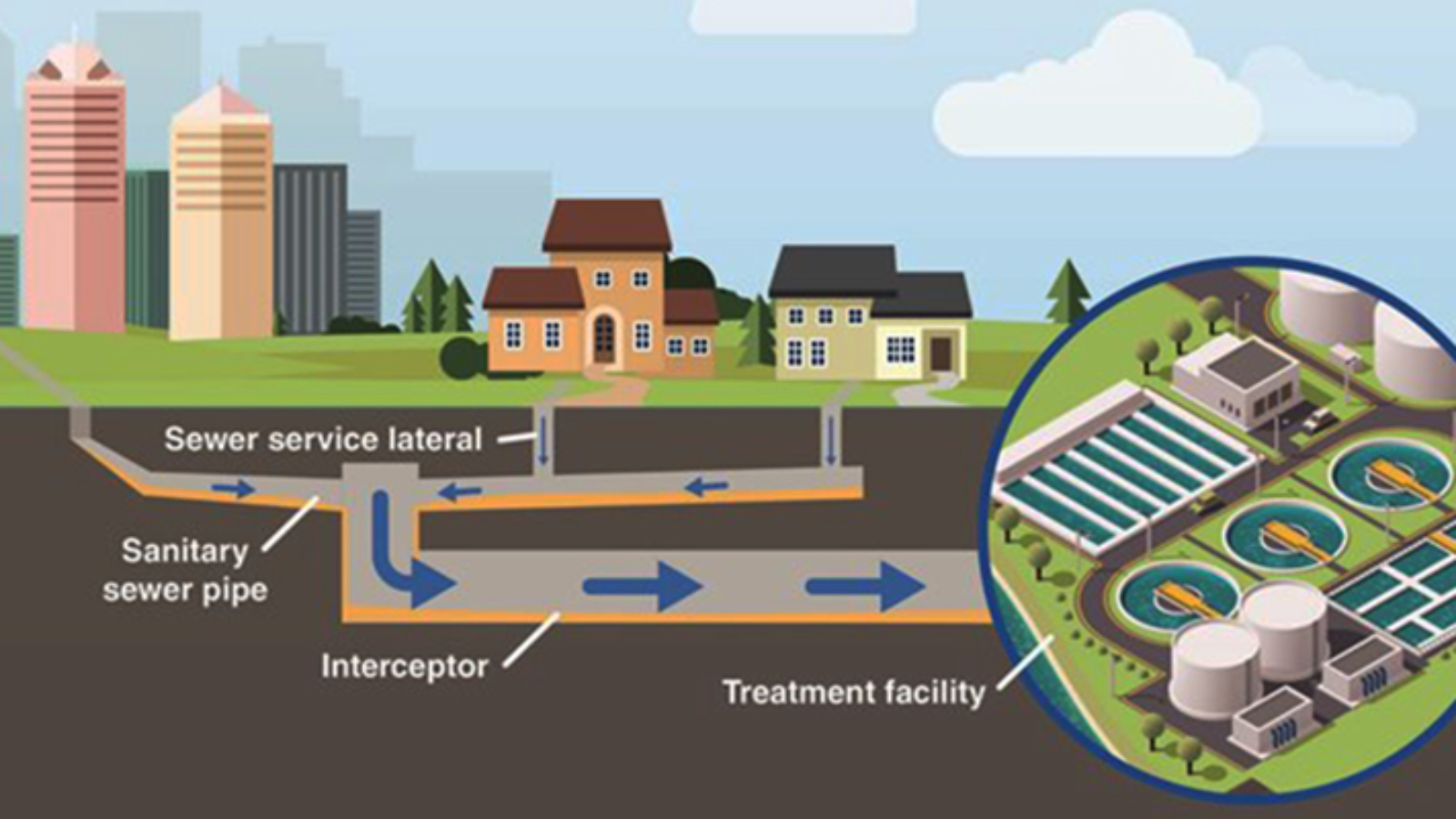
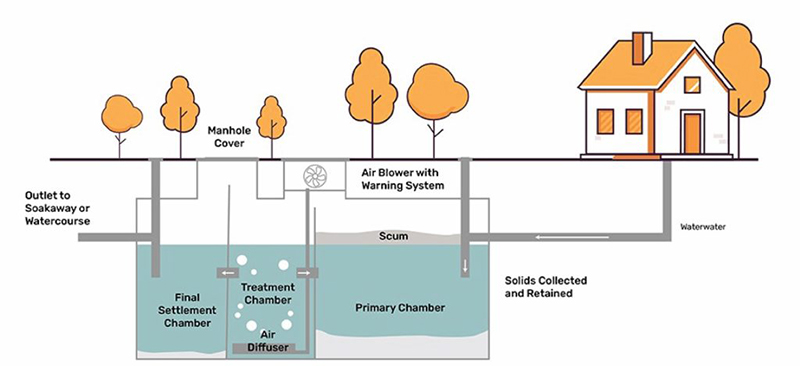
Wastewater treatment is an essential process for industries and municipalities to ensure compliance with environmental regulations and to promote sustainability. Wastewater treatment filters play a crucial role in removing solids, contaminants, and impurities from wastewater before discharge or reuse.
There are several types of wastewater treatment filters, each designed to target specific contaminants and ensure cleaner water output. These filters range from primary filtration systems that remove large debris to advanced filtration technologies that eliminate microscopic particles and dissolved contaminants.
AIMEQUIP provides a range of advanced wastewater treatment filters tailored for industrial applications. This article explores the importance of filtration in wastewater treatment and introduces AIMEQUIP’s specialized filter products that enhance efficiency and performance.
Types of Wastewater Treatment Filters
1- Sand Filtration – Primary Filtration for Removing Suspended Solids
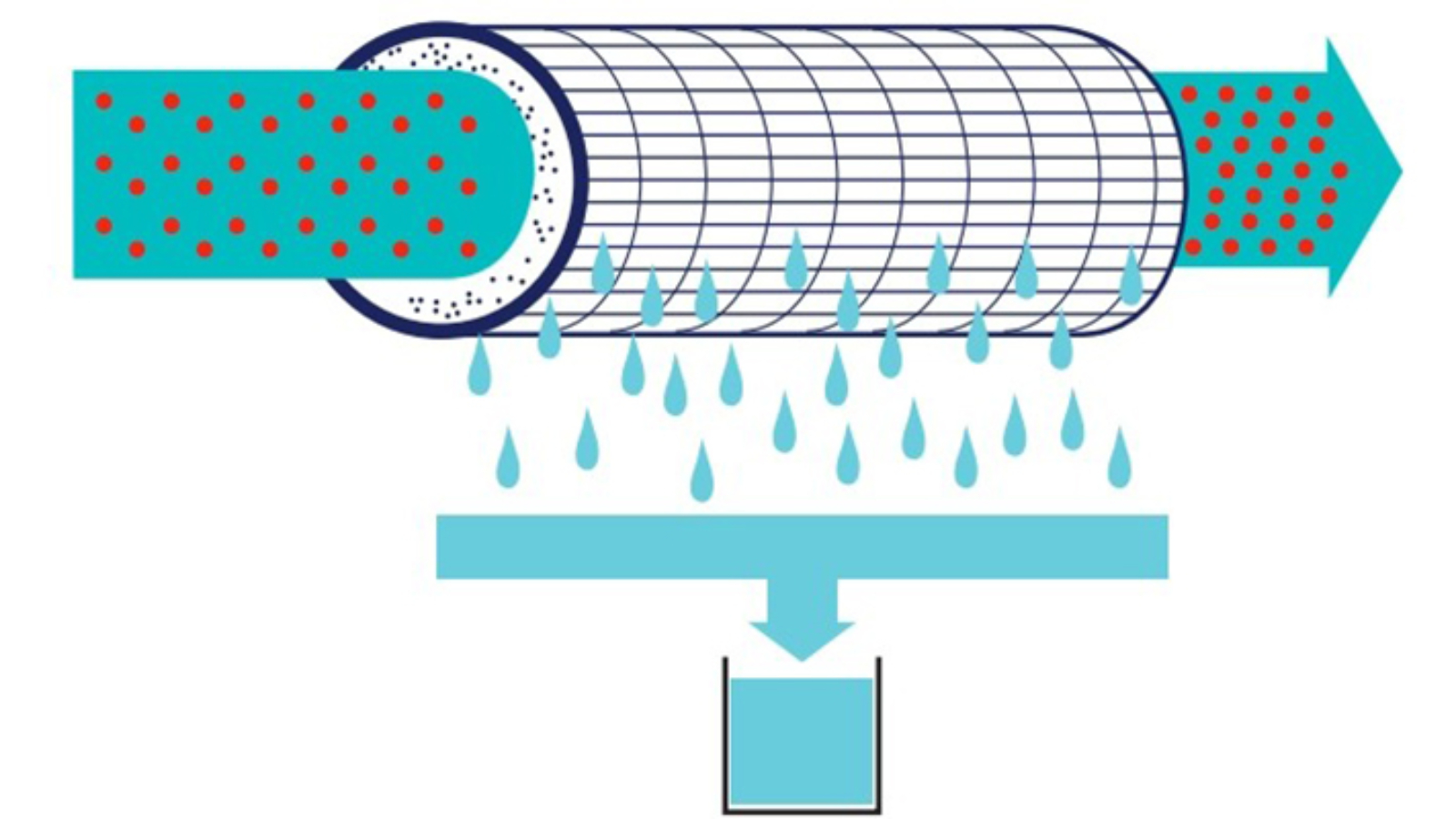
Sand filtration is a widely used method in wastewater treatment filters that involves passing wastewater through a bed of sand to trap and remove large particles and debris. Water flows downward through the sand layers, allowing suspended solids to settle and be retained while cleaner water continues through. Periodic backwashing cleans the sand bed, ensuring continued efficiency. This filtration method is widely used in drinking water treatment, industrial processing, and municipal wastewater treatment plants.
🔹 Key Benefits:
- Simple and cost-effective.
- Handles high flow rates efficiently.
- Requires minimal maintenance.
- Removes large particulates and sediments effectively.
2- Activated Carbon Filtration – Organic Contaminant and Odor Removal
Activated carbon filtration effectively removes organic compounds, chlorine, and other contaminants by adsorption. Water passes through a bed of activated carbon, where pollutants adhere to the porous carbon surface. This method is commonly used in wastewater treatment filters to enhance water quality by effectively removing residual chemicals and improving taste and odor. This filtration is highly effective in treating industrial wastewater, drinking water purification, and air purification systems.
🔹 Key Benefits:
- High adsorption capacity for organic pollutants.
- Reduces residual chemicals and improves water clarity.
- Widely used in industrial and municipal treatment applications.
- Removes volatile organic compounds (VOCs) and disinfection by-products.
Explore Activated Carbon Filter
3- Membrane Filtration – High-Precision Wastewater Processing
Membrane filtration utilizes a semi-permeable barrier to separate contaminants based on their molecular size. Different types of membranes include:
- Microfiltration (MF): Removes large particles and bacteria through membranes with small pore sizes.
- Ultrafiltration (UF): Filters finer pathogens, including viruses, while retaining essential minerals.
- Nanofiltration (NF): Targets specific contaminants such as heavy metals and organic molecules.
- Reverse Osmosis (RO): Uses high pressure to remove dissolved salts, chemicals, and pollutants, producing purified water.
These processes ensure the removal of pollutants while maintaining high water recovery rates in wastewater treatment filters. These membranes are widely used in industries such as pharmaceuticals, food and beverage production, and semiconductor manufacturing.
🔹 Key Benefits:
- Capable of filtering down to microscopic and molecular levels.
- Produces high-purity water suitable for reuse.
- Can be used in both pre-treatment and final treatment stages for water purification.
- Effective in desalination and high-contaminant environments.
4- Biological Filtration – Sustainable Removal of Organic Matter
Biological filtration uses beneficial microorganisms to degrade organic pollutants in wastewater treatment filters. Bacteria and other microbes digest biodegradable contaminants, converting them into harmless byproducts such as carbon dioxide and water. Common biological filters include trickling filters, biofilm reactors, and moving bed bioreactors (MBBR). These systems help reduce biochemical oxygen demand (BOD) and nitrogen levels in water treatment facilities. These are commonly used in municipal wastewater treatment plants and industrial processes where organic waste is prevalent.
🔹 Key Benefits:
- Eco-friendly and sustainable treatment method.
- Effectively removes organic matter and nutrients.
- Can be combined with mechanical and chemical filtration for enhanced results.
- Reduces sludge production compared to conventional treatment methods.
5- Electrocoagulation – Innovative Particle Removal
Electrocoagulation is a cutting-edge technology in wastewater treatment filters that applies electrical currents to wastewater, causing contaminants like heavy metals, oils, and suspended solids to destabilize and clump together for easy removal and separate from the water. The process allows for efficient removal of pollutants without requiring excessive chemical additives. This method is widely used in industries dealing with metal processing, oil and gas, textile manufacturing, and food processing.
🔹 Key Benefits:
- Reduces the need for chemical coagulants.
- Effectively removes a wide range of contaminants.
- Works efficiently in high-flow industrial wastewater treatment systems.
- Reduces heavy metal toxicity and removes complex hydrocarbons.
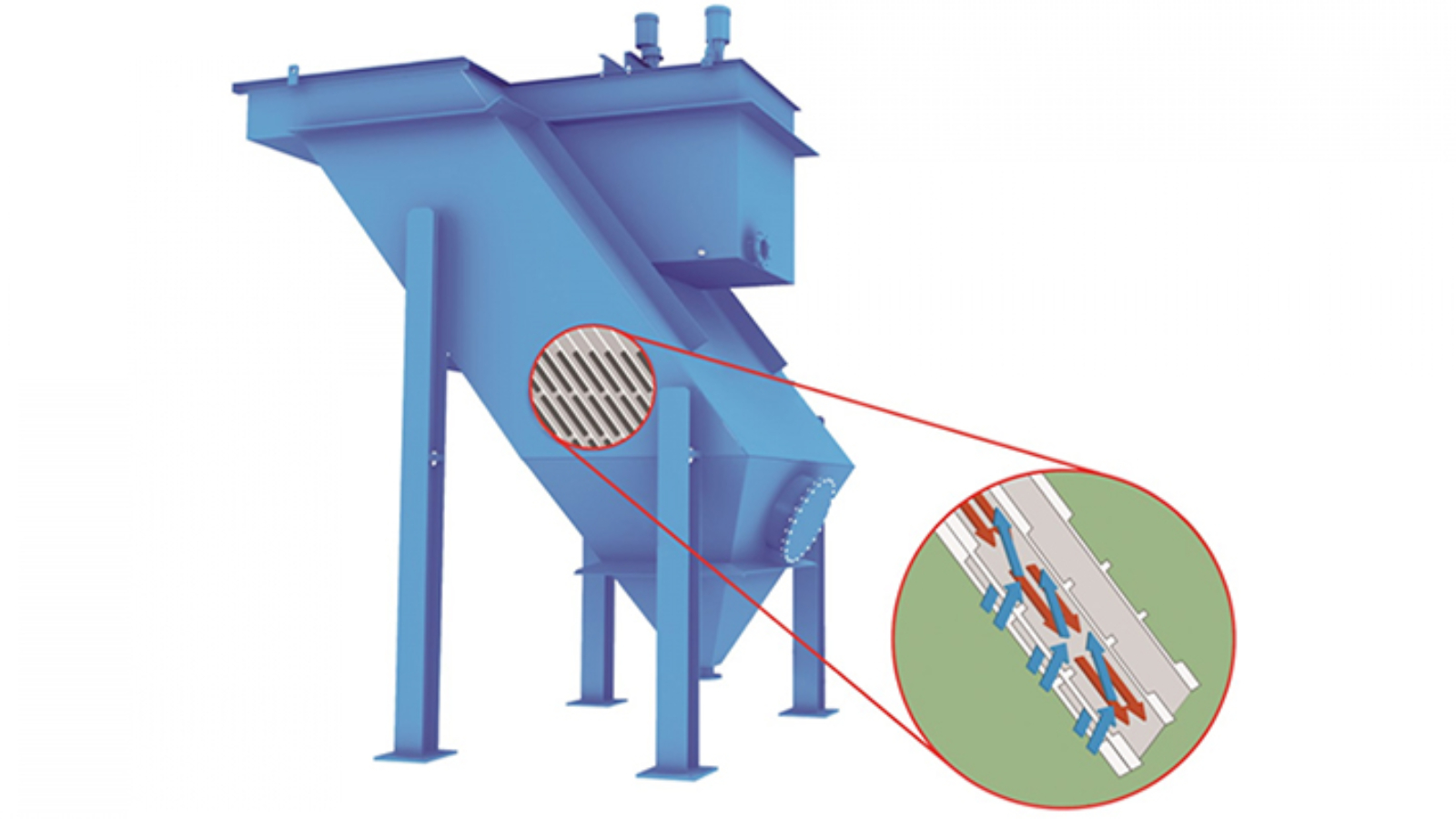
6- Sludge Dewatering – Mechanical Dewatering of Sludge
Sludge dewatering removes excess moisture from wastewater sludge, reducing its volume for easier handling and disposal. This process utilizes mechanical equipment such as belt filter presses, screw presses, and centrifuges to extract water and produce drier sludge cakes. These cakes are easier to transport and dispose of in landfills or recycling processes. Industries with high sludge production, such as pulp and paper, mining, and food processing, rely on dewatering techniques to manage waste efficiently. This step is essential for minimizing waste volume and disposal costs in wastewater treatment filters.
🔹 Key Benefits:
- Reduces overall sludge volume, saving on disposal costs.
- Energy-efficient operation for continuous processing.
- Suitable for industries with high sludge production, such as food processing and mining.
- Enhances sludge management and disposal efficiency.
Explore Belt Filter Press Dewatering
Wastewater treatment filters are essential in ensuring clean and safe water disposal or reuse. Each type of filtration method operates differently, targeting specific contaminants to improve water quality. AIMEQUIP provides specialized solutions and products for various industrial and municipal applications.